Formation Mechanism of the First Carbon–Carbon Bond and the First Olefin in the Methanol Conversion into Hydrocarbons
 |
Yue Liu, S. Müller, D. Berger, J. Jelic, K. Reuter, M. Tonigold, M. Sanchez-Sanchez and J.A. Lercher Angew Chem Intern. Ed., 2016, 55, 5723-5726 DOI: 10.1002/anie.201511678, Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission
|
|---|
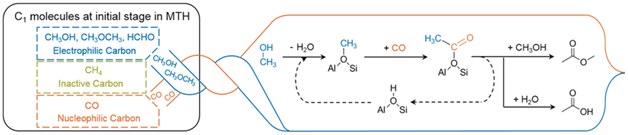
Combining kinetics, spectroscopy, and DFT calculations shows the first carbon–carbon bond in the methanol-to-hydrocarbons reaction is formed through the carbonylation of methanol or dimethyl ether, generating acetic acid and methyl acetate. Olefins arise as secondary products from these acetyl species. Professor Lercher’s group in collaboration with prof. Reuter’s group recently published an article in Angewandte Chemie which can be found at DOI: 10.1002/anie.201511678
(http://onlinelibrary.wiley.com/doi/10.1002/anie.201511678/abstract )