Confinement effects and acid strength in zeolites
 |
|---|
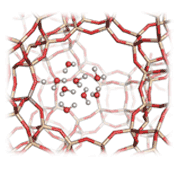
The gradual transition from zeolite Brønsted acid sites to hydronium ions in zeolites of varying pore size is examined, in this collaborative work of ETH, TUM and PNNL groups, by ab initio molecular dynamics combined with enhanced sampling based on Well-Tempered Metadynamics and a recently developed set of collective variables. It was found that while at low water content (1–2 water/BAS) the acidic protons prefer to be shared between zeolites and water, higher water contents (n > 2) invariably lead to solvation of the protons within a localized water cluster adjacent to the BAS. Full publication can be accessed at https://doi.org/10.1038/s41467-021-22936-0