Solvent-determined mechanistic pathways in zeolite-H-BEA-catalysed phenol alkylation
 |
|---|
|
Yuanshuai Liu, Eszter Baráth, Hui Shi, Jianzhi Hu, Donald M. Camaioni, Johannes A. Lercher |
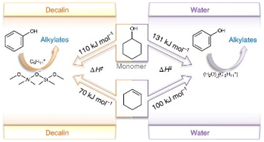
This article is about unravelling how alkylating reactants and solvents significantly alter the reaction pathways of zeolite-catalysed alkylation of phenol in the liquid phase. The carbenium ion formed from the dehydration of cyclohexanol or from the adsorption and protonation of cyclohexene acts as the electrophile, inducing carbon–carbon bond formation. Higher alkylation rates in apolar solvents than in water are caused by the energetically more-favourable carbenium ion formation from either alcohol or olefin on non-hydrated zeolite BAS than on hydronium ions produced by BAS in pores filled with water. The work is a collaboration between Tum and PNNL headed by Prof. Johannes Lercher.
Full article can be found in https://www.nature.com/articles/s41929-017-0015-z#Ack1
Nature Catalysis, volume 1, pages 141–147 (2018)
Reproduced with permission from Springer Nature