Enhancing the catalytic activity of hydronium ions through constrained environments
 |
|---|
|
Y. Liu, A. Vjunov, S. Hui, S. Eckstein, D. Camaioni, D. Mei, E. Baráth, J.A. Lercher |
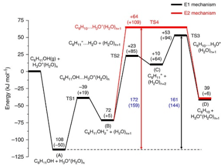
The importance of confined spaces in the rate of catalytic reaction is demonstrated here by the groups at TUM and PNNL. Hydronium ions confined in the nanopores of zeolite HBEA catalyse aqueous phase dehydration of cyclohexanol at a rate significantly higher than hydronium ions in water. This rate enhancement is not related to a shift in mechanism but is caused by the enhanced association between the hydronium ion and the alcohol, as well as a higher intrinsic rate constant in the constrained environments compared with water.
Nature Communications 8, 2017, 14113
DOI: 10.1038/ncomms14113 (open access)
